
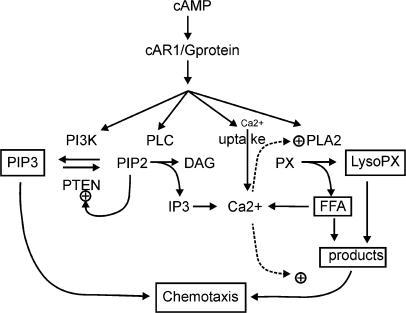
There are three highly related isoforms of Akt (Akt1, Akt2 and Akt3), which phosphorylate substrates containing the consensus phosphorylation motif RxRxxS/T. The frequency with which dysregulated Akt signaling contributes to human disease has culminated in the aggressive development of small molecule inhibitors of PI3K and Akt. Activating mutations in Akt have also been described. In addition, PTEN is frequently mutated or lost in human tumors. In cancer, two mutations that increase the intrinsic kinase activity of PI3K have been identified. In addition, the tumor suppres- sor phosphatase and tensin homolog (PTEN) inhibits Akt activity by dephosphorylating PIP3.ĭysregulation of the PI3K/Akt pathway is implicated in a number of human diseases including cancer, diabetes, cardiovascular disease and neurological diseases. Akt is dephosphorylated by protein phosphatase 2A (PP2A) and the PH-domain leucine-rich-repeat-containing protein phosphatases (PHLPP1/2). Members of the PI3K-related kinase (PIKK) family, including DNA-PK, can also phosphorylate Akt at Ser473.
Pip3 pathway full#
Phosphorylation of Akt at Ser473 by mTORC2 stimulates full enzymatic activity. At the membrane PDK1 phosphorylates Akt at Thr308 leading to partial activation of Akt.
Pip3 pathway Activator#
These lipids serve as plasma membrane docking sites for proteins that harbor pleckstrin-homol- ogy (PH) domains, including Akt and its upstream activator PDK1. The Akt signaling cascade is activated by receptor tyrosine kinases, integrins, B and T cell receptors, cytokine receptors, G-protein-coupled receptors and other stimuli that induce production of phospha- tidylinositol (3,4,5) trisphosphates (PIP3) by phosphoinositide 3-kinase (PI3K). Since its initial discovery as a proto-oncogene, the serine/threonine kinase Akt (also known as protein kinase B or PKB) has become a major focus of attention because of its critical role in regulating diverse cellular functions including metabolism, growth, proliferation, survival, transcription and protein synthesis.


 0 kommentar(er)
0 kommentar(er)
